Zanidatamab GEA Clinical Trial
Zanidatamab GEA Clinical Trial
For U.S. and Canada audiences only
Advanced or Metastatic Gastroesophageal Adenocarcinoma (GEA), Including Stomach, Gastroesophageal Junction, and Esophagus Cancers
Basic eligibility requirements
Adults who have:
- Been diagnosed with a specific type (called HER2-postive) of advanced or metastatic (which means the cancer has spread to other parts of the body) gastroesophageal adenocarcinoma (GEA), including stomach cancer, gastroesophageal junction cancer, and esophagus cancer (adenocarcinoma)
- Not received cancer treatment in the advanced/metastatic setting
- Never received treatments targeting specific proteins called HER2 or PD-1/ PD-L1
Therapies
There will be three treatment groups:
- Zanidatamab (ZW25) in combination with chemotherapy
- Tislelizumab (BGB-A317) in combination with zanidatamab and chemotherapy
- Trastuzumab (Herceptin®) in combination with chemotherapy
Note: Zanidatamab and tislelizumab have not been approved for treatment of GEA
Trial locations
- Recruitment will occur at approximately 300 trial sites in more than 30 countries across Africa, Asia, Europe, North America, Oceania, and South America
HERIZON-GEA-01: A Study of Zanidatamab in Combination With Chemotherapy Plus or Minus Tislelizumab in Patients With a Specific Type of (called HER2-positive) Advanced or Metastatic Gastric and Esophageal Cancers
Status: Recruiting
Clinical Trial: Phase 3
National Clinical Trial (NCT) number: NCT05152147
HERIZON-GEA-01 (also known as the ZW25-301 trial) is a global, randomized, open-label, active-comparator, Phase III study to evaluate and compare the efficacy and safety of zanidatamab plus chemotherapy with or without tislelizumab to the standard of care (trastuzumab plus chemotherapy) as first-line treatment for patients with advanced/metastatic human epidermal growth factor receptor 2 (HER2) positive gastroesophageal adenocarcinomas (GEAs). This study explores chemotherapy combined with either trastuzumab (Herceptin®), zanidatamab, or zanidatamab plus tislelizumab.
Trastuzumab (Herceptin®) is a drug that, when combined with chemotherapy, has been approved in many countries to treat HER2-positive gastric and gastroesophageal cancers. Although not specifically approved for HER2-positive esophageal adenocarcinomas, it is included in global treatment guidelines for this disease.
Zanidatamab (also called ZW25 or JZP598) is a drug that is being explored as an investigational therapy. Zanidatamab is designed to target specific forms of cancer that express a protein called HER2. A doctor can test if a patient’s cancer expresses HER2.
Tislelizumab (also called BGB-A317) is a drug that is being explored as an investigational therapy. Tislelizumab is designed to stimulate the immune system to fight cancer by targeting a protein called PD-1 (short for programmed cell death-1) on certain cells.
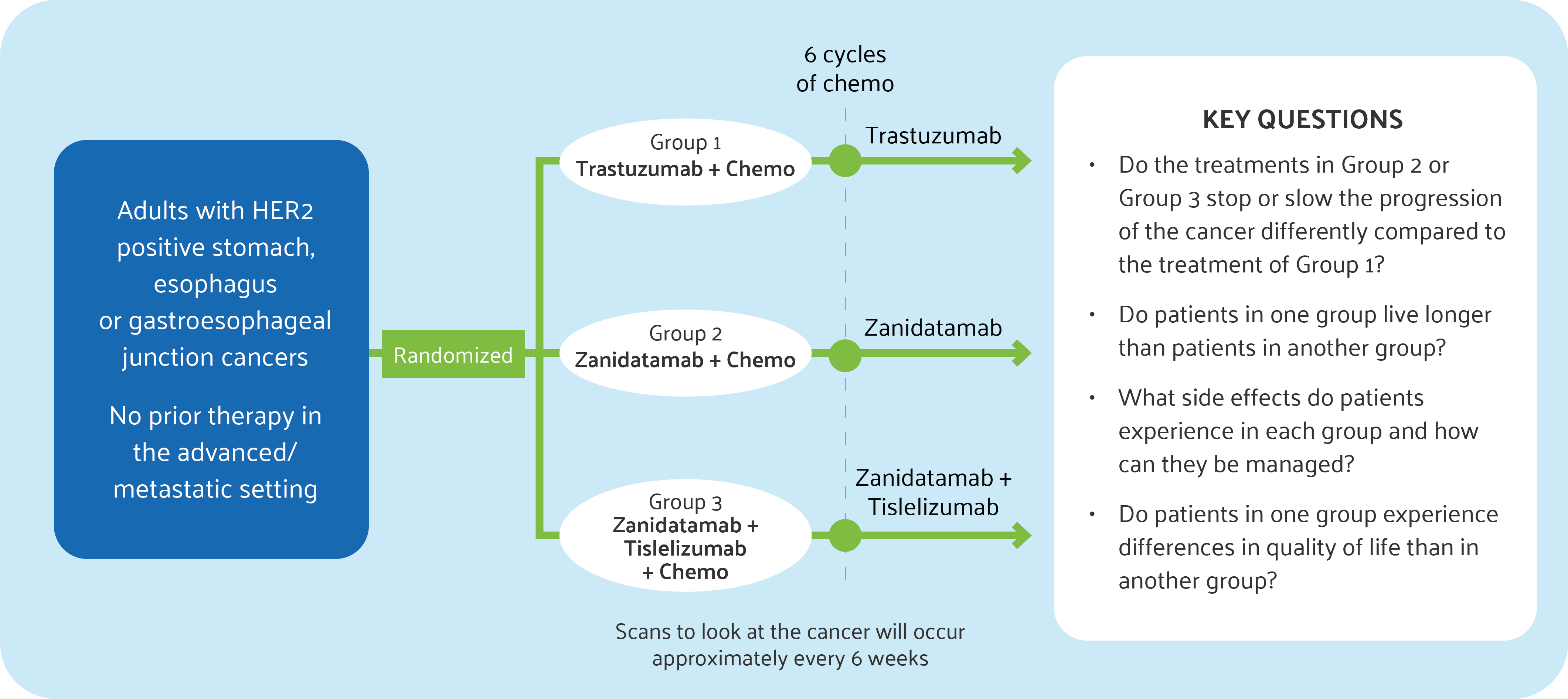
In HERIZON-GEA-01, patients will be randomly assigned to one of three groups:
- Group 1 – Trastuzumab combined with chemotherapy
- Group 2 – Zanidatamab combined with chemotherapy
- Group 3 – Tislelizumab combined with zanidatamab and chemotherapy
All patients will receive treatment by intravenous infusions (also called IV infusions, which is when a needle is inserted into a vein in the arm) every 3 weeks. In addition to IV infusions, some chemotherapy may be provided as a pill. Enrolled patients will also attend visits at their trial medical clinic so that the trial staff can closely monitor and manage their safety and any effects the investigational therapy is having on their cancer.
Trial Purpose
The purpose of the HERIZON-GEA-01 study is to find out if zanidatamab combined with chemotherapy, with or without tislelizumab, works better than trastuzumab (Herceptin®) combined with chemotherapy.
Key Trial Eligibility
To be eligible for this clinical trial, a patient must:
- Be an adult who has been diagnosed with advanced or metastatic HER2-positive gastroesophageal adenocarcinoma (includes stomach cancer, gastroesophageal junction cancer, and esophagus cancer) that is not eligible for curative surgery or procedures. (The patient’s doctor will test a tissue sample to confirm the HER2 status).
- Previous cancer treatments are generally not allowed except under certain conditions. The treating doctor will confirm this.
Additional eligibility criteria will apply. Discuss with your doctor to understand if you are eligible for this trial.
Trial Design
The HERIZON-GEA-01 trial is designed to have approximately 900 patients with HER2-positive gastroesophageal cancer participate.
Patients who choose to participate in HERIZON-GEA-01 will have tests and procedures done throughout the trial. Trial staff will monitor the health of all patients as well as any changes in their cancer.
In this trial, patients will be randomly assigned to one of three groups:
- Group 1 – Trastuzumab combined with chemotherapy
- Group 2 – Zanidatamab combined with chemotherapy
- Group 3 – Tislelizumab combined with zanidatamab and chemotherapy
The image below illustrates a simplified view of the trial design with the key questions the study aims to answer.
Trial Site Locations
Recruitment will occur at approximately 300 trial sites in more than 30 countries across Africa, Asia, Europe, North America, Oceania, and South America
Trial Contact Information
For more information about this trial and participating sites, please contact clinicaltrialdisclosure@jazzpharma.com or visit clinicaltrials.gov (search “NCT05152147” or click here).
